what name is given to a gene that causes cancer
The central aberration resulting in the evolution of cancer is the continual unregulated proliferation of cancer cells. Rather than responding appropriately to the signals that control normal cell behavior, cancer cells grow and divide in an uncontrolled way, invading normal tissues and organs and eventually spreading throughout the body. The generalized loss of growth control exhibited by cancer cells is the cyberspace result of accumulated abnormalities in multiple cell regulatory systems and is reflected in several aspects of prison cell behavior that distinguish cancer cells from their normal counterparts.
Types of Cancer
Cancer tin effect from abnormal proliferation of whatsoever of the dissimilar kinds of cells in the body, so there are more than than a hundred singled-out types of cancer, which tin can vary substantially in their behavior and response to treatment. The nearly important result in cancer pathology is the distinction between benign and malignant tumors (Figure 15.1). A tumor is any abnormal proliferation of cells, which may exist either benign or malignant. A benign tumor, such as a common skin wart, remains confined to its original location, neither invading surrounding normal tissue nor spreading to afar body sites. A malignant tumor, nevertheless, is capable of both invading surrounding normal tissue and spreading throughout the trunk via the circulatory or lymphatic systems (metastasis). Only malignant tumors are properly referred to as cancers, and it is their ability to invade and metastasize that makes cancer and so unsafe. Whereas benign tumors tin usually be removed surgically, the spread of cancerous tumors to distant body sites ofttimes makes them resistant to such localized treatment.

Figure 15.1
A cancerous tumor of the uterus. Micrographs of normal uterus (A) and a section of a uterine sarcoma (B). Annotation that the cancer cells (darkly stained) have invaded the surrounding normal tissue. (Cecil Play a joke on/Molecular Histology, Inc.)
Both beneficial and malignant tumors are classified co-ordinate to the type of cell from which they ascend. Most cancers autumn into one of three master groups: carcinomas, sarcomas, and leukemias or lymphomas. Carcinomas, which include approximately xc% of human cancers, are malignancies of epithelial cells. Sarcomas, which are rare in humans, are solid tumors of connective tissues, such as muscle, os, cartilage, and fibrous tissue. Leukemias and lymphomas, which account for approximately eight% of human malignancies, arise from the blood-forming cells and from cells of the immune system, respectively. Tumors are farther classified according to tissue of origin (e.k., lung or breast carcinomas) and the type of cell involved. For instance, fibrosarcomas ascend from fibroblasts, and erythroid leukemias from precursors of erythrocytes (scarlet blood cells).
Although there are many kinds of cancer, only a few occur ofttimes (Table xv.1). More than a million cases of cancer are diagnosed annually in the United States, and more than 500,000 Americans dice of cancer each year. Cancers of 10 unlike body sites account for more than 75% of this total cancer incidence. The four most common cancers, accounting for more than half of all cancer cases, are those of the breast, prostate, lung, and colon/rectum. Lung cancer, by far the most lethal, is responsible for nearly 30% of all cancer deaths.
Table 15.1
Ten Nigh Frequent Cancers in the United States.
The Development of Cancer
Ane of the central features of cancer is tumor clonality, the development of tumors from single cells that brainstorm to proliferate abnormally. The single-cell origin of many tumors has been demonstrated by analysis of X chromosome inactivation (Figure xv.2). As discussed in Chapter viii, ane member of the Ten chromosome pair is inactivated by being converted to heterochromatin in female person cells. 10 inactivation occurs randomly during embryonic development, and then one Ten chromosome is inactivated in some cells, while the other X chromosome is inactivated in other cells. Thus, if a female person is heterozygous for an 10 chromosome gene, dissimilar alleles will be expressed in different cells. Normal tissues are composed of mixtures of cells with different inactive X chromosomes, so expression of both alleles is detected in normal tissues of heterozygous females. In contrast, tumor tissues generally express only ane allele of a heterozygous X chromosome gene. The implication is that all of the cells constituting such a tumor were derived from a single cell of origin, in which the pattern of 10 inactivation was fixed before the tumor began to develop.
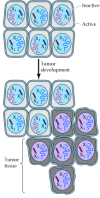
Figure xv.2
Tumor clonality. Normal tissue is a mosaic of cells in which dissimilar X chromosomes (101 and 10two) have been inactivated. Tumors develop from a single initially altered cell, so each tumor cell displays the same pattern of X inactivation (101 inactive, X (more than...)
The clonal origin of tumors does not, however, imply that the original progenitor jail cell that gives rise to a tumor has initially acquired all of the characteristics of a cancer cell. On the contrary, the development of cancer is a multistep process in which cells gradually become malignant through a progressive series of alterations. One indication of the multistep development of cancer is that nearly cancers develop late in life. The incidence of colon cancer, for example, increases more than tenfold between the ages of xxx and 50, and another tenfold between 50 and 70 (Effigy 15.3). Such a dramatic increase of cancer incidence with age suggests that virtually cancers develop as a consequence of multiple abnormalities, which accumulate over periods of many years.

Figure 15.3
Increased rate of colon cancer with age. Annual expiry rates from colon cancer in the U.s.. (Information from J. Cairns, 1978. Cancer: Science and Society, New York: W. H. Freeman.)
At the cellular level, the development of cancer is viewed as a multistep procedure involving mutation and selection for cells with progressively increasing chapters for proliferation, survival, invasion, and metastasis (Figure fifteen.4). The get-go footstep in the process, tumor initiation, is thought to be the effect of a genetic amending leading to abnormal proliferation of a unmarried cell. Cell proliferation then leads to the outgrowth of a population of clonally derived tumor cells. Tumor progression continues as additional mutations occur inside cells of the tumor population. Some of these mutations confer a selective advantage to the cell, such as more than rapid growth, and the descendants of a prison cell begetting such a mutation will consequently become ascendant within the tumor population. The process is called clonal pick, since a new clone of tumor cells has evolved on the basis of its increased growth rate or other properties (such as survival, invasion, or metastasis) that confer a selective advantage. Clonal selection continues throughout tumor development, so tumors continuously go more rapid-growing and increasingly malignant.

Figure fifteen.4
Stages of tumor development. The development of cancer initiates when a unmarried mutated jail cell begins to proliferate abnormally. Boosted mutations followed by selection for more rapidly growing cells within the population then result in progression of (more...)
Studies of colon carcinomas have provided a clear case of tumor progression during the evolution of a common human malignancy (Figure fifteen.5). The earliest stage in tumor evolution is increased proliferation of colon epithelial cells. One of the cells within this proliferative jail cell population is then thought to requite rising to a small benign neoplasm (an adenoma or polyp). Further rounds of clonal selection lead to the growth of adenomas of increasing size and proliferative potential. Cancerous carcinomas then ascend from the benign adenomas, indicated past invasion of the tumor cells through the basal lamina into underlying connective tissue. The cancer cells then continue to proliferate and spread through the connective tissues of the colon wall. Eventually the cancer cells penetrate the wall of the colon and invade other abdominal organs, such equally the float or small intestine. In add-on, the cancer cells invade blood and lymphatic vessels, allowing them to metastasize throughout the body.
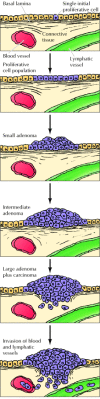
Figure 15.5
Evolution of colon carcinomas. A single initially contradistinct prison cell gives ascension to a proliferative jail cell population, which progresses first to benign adenomas of increasing size and then to malignant carcinoma. The cancer cells invade the underlying connective (more...)
Causes of Cancer
Substances that cause cancer, chosen carcinogens, have been identified both past studies in experimental animals and by epidemiological analysis of cancer frequencies in human populations (e.g., the high incidence of lung cancer among cigarette smokers). Since the development of malignancy is a circuitous multistep process, many factors may bear on the likelihood that cancer will develop, and it is overly simplistic to speak of unmarried causes of about cancers. Even so, many agents, including radiation, chemicals, and viruses, take been found to induce cancer in both experimental animals and humans.
Radiations and many chemical carcinogens (Figure xv.half-dozen) act by damaging DNA and inducing mutations. These carcinogens are more often than not referred to as initiating agents, since the induction of mutations in key target genes is thought to be the initial issue leading to cancer development. Some of the initiating agents that contribute to human cancers include solar ultraviolet radiations (the major crusade of skin cancer), carcinogenic chemicals in tobacco smoke, and aflatoxin (a stiff liver carcinogen produced past some molds that contaminate improperly stored supplies of peanuts and other grains). The carcinogens in tobacco smoke (including benzo(a)pyrene, dimethylnitrosamine, and nickel compounds) are the major identified causes of human being cancer. Smoking is the undisputed cause of 80 to ninety% of lung cancers, as well as existence implicated in cancers of the oral crenel, pharynx, larynx, esophagus, and other sites. In total, it is estimated that smoking is responsible for virtually i-tertiary of all cancer deaths—an impressive toll for a single carcinogenic agent.

Figure xv.6
Structure of representative chemic carcinogens.
Other carcinogens contribute to cancer development by stimulating cell proliferation, rather than by inducing mutations. Such compounds are referred to every bit tumor promoters, since the increased cell division they induce is required for the outgrowth of a proliferative cell population during early stages of tumor development. The phorbol esters that stimulate prison cell proliferation by activating protein kinase C (meet Figure 13.26) are classic examples. Their activeness was divers by studies of chemical induction of skin tumors in mice (Figure 15.7). Tumorigenesis in this organisation can be initiated by a unmarried handling with a mutagenic carcinogen. Tumors do non develop, however, unless the mice are afterward treated with a tumor promoter (ordinarily a phorbol ester) to stimulate proliferation of the mutated cells.
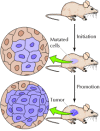
Effigy xv.7
Induction of tumors in mouse skin. Tumors are initiated past mutations induced past a carcinogen. Development of a tumor then requires treatment with a tumor promoter to stimulate proliferation of the mutated cells.
Hormones, particularly estrogens, are of import as tumor promoters in the evolution of some homo cancers. The proliferation of cells of the uterine endometrium, for instance, is stimulated by estrogen, and exposure to excess estrogen significantly increases the likelihood that a woman volition develop endometrial cancer. The adventure of endometrial cancer is therefore substantially increased past long-term postmenopausal estrogen replacement therapy with loftier doses of estrogen lone. Fortunately, this risk is minimized by administration of progesterone to counteract the stimulatory effect of estrogen on endometrial jail cell proliferation. However, long-term therapy with combinations of estrogen and progesterone may lead to an increased take a chance of breast cancer.
In improver to chemicals and radiation, some viruses induce cancer both in experimental animals and in humans. The common human cancers caused past viruses include liver cancer and cervical carcinoma, which together account for 10 to twenty% of worldwide cancer incidence. These viruses are of import not merely as causes of human cancer; as discussed later on in this affiliate, studies of tumor viruses have played a key role in elucidating the molecular events responsible for the development of cancers induced past both viral and nonviral carcinogens.
Properties of Cancer Cells
The uncontrolled growth of cancer cells results from accumulated abnormalities affecting many of the cell regulatory mechanisms that accept been discussed in preceding chapters. This relationship is reflected in several aspects of cell beliefs that distinguish cancer cells from their normal counterparts. Cancer cells typically display abnormalities in the mechanisms that regulate normal cell proliferation, differentiation, and survival. Taken together, these characteristic backdrop of cancer cells provide a clarification of malignancy at the cellular level.
The uncontrolled proliferation of cancer cells in vivo is mimicked by their behavior in cell culture. A primary distinction between cancer cells and normal cells in culture is that normal cells display density-dependent inhibition of cell proliferation (Figure 15.8). Normal cells proliferate until they attain a finite cell density, which is determined in part past the availability of growth factors added to the culture medium (ordinarily in the class of serum). They then cease proliferating and get quiescent, arrested in the K0 stage of the cell bicycle (see Figure fourteen.half-dozen). The proliferation of near cancer cells, even so, is not sensitive to density-dependent inhibition. Rather than responding to the signals that crusade normal cells to cease proliferation and enter Thousand0, tumor cells generally go along growing to high cell densities in civilization, mimicking their uncontrolled proliferation in vivo.

Figure 15.8
Density-dependent inhibition. Normal cells proliferate in culture until they reach a finite cell density, at which signal they get quiescent. Tumor cells, however, continue to proliferate independent of cell density.
A related difference between normal cells and cancer cells is that many cancer cells accept reduced requirements for extracellular growth factors. As discussed in Chapter 13, the proliferation of about cells is controlled, at least in part, by polypeptide growth factors. For some cell types, particularly fibroblasts, the availability of serum growth factors is the principal determinant of their proliferative capacity in culture. The growth factor requirements of these cells are closely related to the phenomenon of density-dependent inhibition, since the density at which normal fibroblasts become quiescent is proportional to the concentration of serum growth factors in the culture medium.
The growth factor requirements of many tumor cells are reduced compared to their normal counterparts, contributing to the unregulated proliferation of tumor cells both in vitro and in vivo. In some cases, cancer cells produce growth factors that stimulate their ain proliferation (Figure 15.nine). Such abnormal production of a growth cistron by a responsive cell leads to continuous autostimulation of prison cell division (autocrine growth stimulation), and the cancer cells are therefore less dependent on growth factors from other, physiologically normal sources. In other cases, the reduced growth factor dependence of cancer cells results from abnormalities in intracellular signaling systems, such equally unregulated activity of growth gene receptors or other proteins (e.g., Ras proteins or protein kinases) that were discussed in Affiliate xiii equally elements of signal transduction pathways leading to cell proliferation.

Figure fifteen.9
Autocrine growth stimulation. A prison cell produces a growth factor to which information technology also responds, resulting in continuous stimulation of cell proliferation.
Cancer cells are also less stringently regulated than normal cells past cell-cell and jail cell-matrix interactions. Most cancer cells are less adhesive than normal cells, often as a result of reduced expression of cell surface adhesion molecules. For instance, loss of E-cadherin, the chief adhesion molecule of epithelial cells, is important in the development of carcinomas (epithelial cancers). As a outcome of reduced expression of cell adhesion molecules, cancer cells are comparatively unrestrained by interactions with other cells and tissue components, contributing to the ability of malignant cells to invade and metastasize. The reduced adhesiveness of cancer cells besides results in morphological and cytoskeletal alterations: Many tumor cells are rounder than normal, in part because they are less firmly attached to either the extracellular matrix or neighboring cells.
A striking deviation in the cell-prison cell interactions displayed by normal cells and those of cancer cells is illustrated past the phenomenon of contact inhibition (Figure fifteen.ten). Normal fibroblasts migrate across the surface of a civilization dish until they brand contact with a neighboring cell. Further cell migration is then inhibited, and normal cells adhere to each other, forming an orderly array of cells on the culture dish surface. Tumor cells, in dissimilarity, keep moving after contact with their neighbors, migrating over next cells, and growing in disordered, multilayered patterns. Not just the motion but likewise the proliferation of many normal cells is inhibited by jail cell-cell contact, and cancer cells are characteristically insensitive to such contact inhibition of growth.

Effigy 15.10
Contact inhibition. Light micrographs (left) and scanning electron micrographs (right) of normal fibroblasts and tumor cells. The migration of normal fibroblasts is inhibited past cell contact, so they form an orderly side-by-side array on the surface of (more...)
Two additional properties of cancer cells touch their interactions with other tissue components, thereby playing of import roles in invasion and metastasis. First, malignant cells mostly secrete proteases that assimilate extracellular matrix components, allowing the cancer cells to invade adjacent normal tissues. Secretion of collagenase, for example, appears to be an important determinant of the ability of carcinomas to digest and penetrate through basal laminae to invade underlying connective tissue (see Figure xv.5). Second, cancer cells secrete growth factors that promote the formation of new blood vessels (angiogenesis). Angiogenesis is needed to back up the growth of a tumor across the size of near a million cells, at which betoken new blood vessels are required to supply oxygen and nutrients to the proliferating tumor cells. Such blood vessels are formed in response to growth factors, secreted by the tumor cells, that stimulate proliferation of endothelial cells in the walls of capillaries in surrounding tissue, resulting in the outgrowth of new capillaries into the tumor. The formation of such new claret vessels is important not only in supporting tumor growth, but also in metastasis. The actively growing new capillaries formed in response to angiogenic stimulation are easily penetrated past the tumor cells, providing a ready opportunity for cancer cells to enter the circulatory system and begin the metastatic procedure.
Some other general characteristic of near cancer cells is that they fail to differentiate normally. Such lacking differentiation is closely coupled to abnormal proliferation, since, as discussed in Chapter 14, most fully differentiated cells either cease jail cell division or divide only rarely. Rather than conveying out their normal differentiation program, cancer cells are normally blocked at an early stage of differentiation, consistent with their continued active proliferation.
The leukemias provide a peculiarly proficient example of the relationship between lacking differentiation and malignancy. All of the different types of blood cells are derived from a common stem cell in the bone marrow (see Figure 14.44). Descendants of these cells so become committed to specific differentiation pathways. Some cells, for example, differentiate to form erythrocytes whereas others differentiate to form lymphocytes, granulocytes, or macrophages. Cells of each of these types undergo several rounds of division as they differentiate, but once they become fully differentiated, cell segmentation ceases. Leukemic cells, in dissimilarity, fail to undergo concluding differentiation (Figure fifteen.11). Instead, they get arrested at early stages of maturation at which they retain their capacity for proliferation and continue to reproduce.
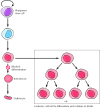
Figure 15.11
Defective differentiation and leukemia. Dissimilar types of claret cells develop from a multipotential (pluripotent) stem cell in the os marrow. The precursors of differentiated cells undergo several rounds of prison cell division as they mature, but prison cell sectionalization (more...)
As discussed in Chapter 13, programmed cell expiry, or apoptosis, is an integral function of the differentiation program of many jail cell types, including blood cells. Many cancer cells fail to undergo apoptosis, and therefore exhibit increased life spans compared to their normal counterparts. This failure of cancer cells to undergo programmed prison cell death contributes substantially to tumor evolution. For instance, the survival of many normal cells is dependent on signals from growth factors or from the extracellular matrix that preclude apoptosis. In contrast, tumor cells are oft able to survive in the absence of growth factors required by their normal counterparts. Such a failure of tumor cells to undergo apoptosis when deprived of normal environmental signals may exist important non but in main tumor development merely also in the survival and growth of metastatic cells in abnormal tissue sites. Normal cells also undergo apoptosis following Dna damage, while many cancer cells fail to do so. In this case, the failure to undergo apoptosis contributes to the resistance of cancer cells to irradiation and many chemotherapeutic drugs, which act by dissentious DNA. Abnormal cell survival, as well every bit prison cell proliferation, thus plays a major part in the unrelenting growth of cancer cells in an beast.
Transformation of Cells in Culture
The study of tumor consecration past radiation, chemicals, or viruses requires experimental systems in which the furnishings of a carcinogenic agent can be reproducibly observed and quantitated. Although the action of carcinogens can be assayed in intact animals, such experiments are difficult to quantitate and control. The development of in vitro assays to detect the conversion of normal cells to tumor cells in civilisation, a process called cell transformation, therefore represented a major advance in cancer research. Such assays are designed to detect transformed cells, which display the in vitro growth properties of tumor cells, following exposure of a culture of normal cells to a carcinogenic agent. Their application has immune experimental analysis of cell transformation to reach a level of sophistication that could not have been attained by studies in whole animals lonely.
The first and most widely used assay of cell transformation is the focus assay, which was developed by Howard Temin and Harry Rubin in 1958. The focus assay is based on the ability to recognize a grouping of transformed cells as a morphologically distinct "focus" against a background of normal cells on the surface of a civilization dish (Figure 15.12). The focus assay takes reward of iii properties of transformed cells: contradistinct morphology, loss of contact inhibition, and loss of density-dependent inhibition of growth. The result is the germination of a colony of morphologically contradistinct transformed cells that overgrow the background of normal cells in the civilisation. Such foci of transformed cells tin usually exist detected and quantified within a calendar week or two subsequently exposure to a carcinogenic agent. In general, cells transformed in vitro are able to form tumors following inoculation into susceptible animals, supporting in vitro transformation every bit a valid indicator of the germination of cancer cells.

Figure 15.12
The focus assay. A focus of craven embryo fibroblasts induced by Rous sarcoma virus. (From H. M. Temin and H. Rubin, 1958. Virology 6: 669.)
Source: https://www.ncbi.nlm.nih.gov/books/NBK9963/
0 Response to "what name is given to a gene that causes cancer"
Post a Comment